Spravato
Treatment-Resistant Depression (TRD)
Proven efficacy with safety evaluated in over 1700 adults with treatment-resistant depression (TRD) across six studies: Five Phase 3 studies and one Phase 2 dose ranging study
Why SPRAVATO
If you’ve taken two or more oral antidepressants and still experience symptoms of depression, you might have treatment-resistant depression (TRD).

REMS-Certified SPRAVATO® Treatment Center Communications Toolkit
What is in the toolkit
Communicating About Your REMSCertified SPRAVATO® Treatment Center
The resources in this section are intended to help you introduce the availability of SPRAVATO® through your treatment center to referring HCPs and patients.
You may use these resources to communicate only about your treatment center’s SPRAVATO®related services.
Communicating About SPRAVATO® and the Patient Experience
The resources in this section are intended to help you stay connected with your referral network and educate patients and HCPs.
Additional Resources
The resources in this section may be useful to have on hand when communicating about SPRAVATO®. Resources include the product logo, Prescribing Information, and Important Safety Information. Please follow all compliance reminders when using these resources.
Please note: This toolkit and its materials are being provided for your conditional use, subject to agreement with these terms. The information in this toolkit is meant to be educational to help inform your communications. Your use of these materials is not an endorsement by Janssen of your treatment center. In no event is Janssen responsible for your use of this material. Modifications are prohibited and Janssen has no liability for any modifications made. Your use of this material does not provide you with any right or license to any of Janssen’s intellectual property, including, without limitation, any Janssen logos, trademarks or images. Janssen reserves all rights under law with respect to its intellectual property. SPRAVATO® is a registered trademark of Johnson & Johnson and its affiliated companies. HCP=healthcare provider; REMS=Risk Evaluation and Mitigation Strategy.
HCPs
When communicating with HCPs about your treatment center, consider using these resources to help plan your HCP engagements, whether they are in person or via email.
An email template introducing your treatment center to potential referring HCPs
A resource to assist with planning and hosting engagement events at your treatment center
A resource to help plan communications and outreach to your referral community
Patients
When communicating with patients about your treatment center, consider using these resources to help guide your communications to be comprehensive and patient-friendly.
An email template introducing your treatment center to potential patients
An educational resource to help onboard patients to the SHARE patient story volunteer program
Community
When engaging with your community, you may use these resources to help build awareness of the availability of SPRAVATO® at your treatment center.
A resource to answer questions about treatment center communications, including guidance on the development of videos, social media content, and promotional materials
A resource to answer questions about treatment center communications, including guidance on the development of videos, social media content, and promotional materials
HCPs
These resources may help you introduce SPRAVATO® to HCPs in your community and keep an open line of communication with your referral network.
A form for HCPs to refer patients to your treatment center
A form for your treatment center to stay in communication with your referring HCPs
Patients
The following resources may assist you in introducing SPRAVATO® to patients in your community. By educating patients about SPRAVATO®, you may help them learn more about what their experience in your treatment center might be like. These resources may also be shared with HCPs for their use with patients.
A video explaining the SPRAVATO® experience to patients from the perspective of experienced prescribers
Patient Videos
Patient testimonial videos for patients to hear firsthand experiences with SPRAVATO®
A video advertisement in the current Going in Circles campaign that promotes SPRAVATO® for the treatment of TRD
Additional Resources
You may use the following resources when communicating about SPRAVATO®. Including the product logo, product image, and description will help ensure that communications about SPRAVATO® are compliant. When communicating about SPRAVATO®, it is important to always include the Healthcare Professional version of the Indications and Important Safety Information (ISI) for internal communications and the Consumer Indications and ISI for external communications
The following materials are available by request from your Janssen representative:
• SPRAVATO® brand presentation
• Customizable patient brochure
• Branded wall cling for your waiting room
• Unbranded wall cling for your treatment center offices
Visit SPRAVATOHCP.com or contact your local SPRAVATO® representative for more information.
How SPRAVATO® Works
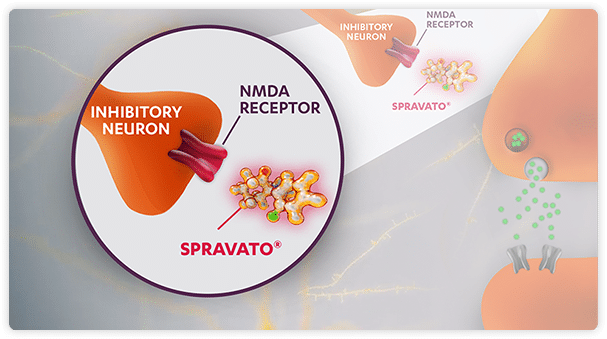
SPRAVATO® is different because it acts on the glutamate pathway
The primary antidepressant activity of SPRAVATO® is not believed to directly involve inhibition of serotonin or norepinephrine reuptake.
The precise mechanism of action (MOA) is unknown.
SPRAVATO® is an NMDA receptor antagonist. An NMDA receptor is an ionotropic glutamate receptor.
SPRAVATO® hypothesized MOA.
NMDA=N-methyl-D-aspartate.
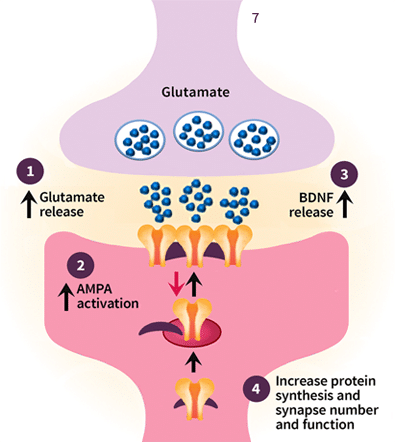
- Clinical study results suggest that glutamate transmission is abnormally regulated in multiple areas of the brain of individuals with depression.
- Antagonism of the NMDA receptor and AMPA receptor activation leads to downstream release of BDNF and activation of kinases.
- BDNF expression and downstream signaling are thought to enhance neurogenesis and mediation of synaptic excitability and plasticity.
- It is important to note that the relationship between this pathway and the clinical effects of depression have not been established.
AMPA=α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid.
BDNF=brain-derived neurotrophic factor.
Safety
Serious side effects of SPRAVATO® include feeling sleepy (sedation or loss of consciousness); feeling disconnected from yourself, your thoughts, feelings and things around you (dissociation); breathing problems (respiratory depression and respiratory arrest); abuse and misuse; increased risk of suicidal thoughts and behavior; increased blood pressure; problems with thinking clearly and bladder problems.
6-Year Clinical Study Results
Please review the following information on how the study was designed and the limitations of the study before viewing the 6-year data.
The following data is from a, Phase 3, open-label, long-term safety extension study in adults with treatment-resistant depression (TRD).
Limitations:
The patients and physicians were aware of the treatment being given, and there was no comparator group. Statistical analyses were not done for the efficacy data, which means no conclusions can be drawn from the results
The results from this study may not represent what every patient may experience. This study only included patients who chose to continue from the previous parent study and the patients in the study did not have two or more significant co-existing diseases (psychiatric or medical, or substance dependence). In addition, the study size got smaller later on in the clinical trial
TRD long-term safety extension study
Study Design
A subgroup analysis was conducted on a cohort of 1,021 patients who met criteria consistent with the on-label population.
Patients in this subgroup analysis were between 18-64 years of age and received SPRAVATO® 56 mg or 84 mg twice weekly during induction (IND) phase and flexible dosing during optimization/maintenance (OP/M) phase; all patients should have taken an approved oral antidepressant throughout the study
The primary purpose of this study was to determine how many patients experienced treatment-emergent adverse events (TEAEs) over time while on SPRAVATO®
Safety Results
- 94.7% of patients experienced ≥1 TEAE
184 patients (18.0%) experienced serious TEAEs - Six deaths (0.6%) related to TEAEs
- occurred; investigator assessment determined none were considered to be related to SPRAVATO®
- A total of 74 patients (7.2.%) experienced one or more TEAE related to suicide
are you in crisis? Get help now!
NEW PATIENT INFORMATION

new patient forms
Before you visit us for your first session, let’s save you some time. Our forms can be accessed online and filled out from the convenience of your own home. Fill them out now so you are all set when you come in.

frequently Asked Questions
Want answers now? Wondering what all these abbreviations mean? Wondering how it all works? Wondering what a specific type of therapy is all about?
We’ve gathered a thorough list of explanations for you here.

suggested readings
Over the years our staff has collected a list of the best books on relavent topics. Many of these books will help you and become a powerful supplement as you talk to your therapist.


